Abstract
Background Early trials of long-term lenalidomide (len) use reported an increased incidence of second primary malignancies (SPM), including acute myeloid leukaemia and myelodysplastic syndrome. Later, meta-analysis suggested the link to be secondary to len in combination with melphalan. Here we address the impact of len on SPM development when used at induction and maintenance, profiling SPM type and incidence in 4358 patients treated in the UK NCRI Myeloma XI trial, in which len was used at induction and maintenance, in transplant eligible (TE) and non-eligible (TNE) newly diagnosed patients.
Methods Myeloma XI was a phase III, randomised, multi-centre, parallel group design, open-label trial comparing cyclophosphamide, thalidomide, len, carfilzomib and bortezomib induction combinations and len as maintenance treatment in newly diagnosed patients. TE patients received high dose melphalan supported by autologous stem cell transplantation. Patients in both pathways were randomised to maintenance with len (+/- vorinostat) or active observation. Within the TE pathway, 701 patients did not receive len at induction or maintenance, 1263 received len at induction or maintenance (single exposure) and 568 received len at induction and maintenance (double exposure). In TNE patients, 677 were not treated with len, 899 were single exposed and 260 double exposed. Median follow-up was 68 months (IQ range 49-83).
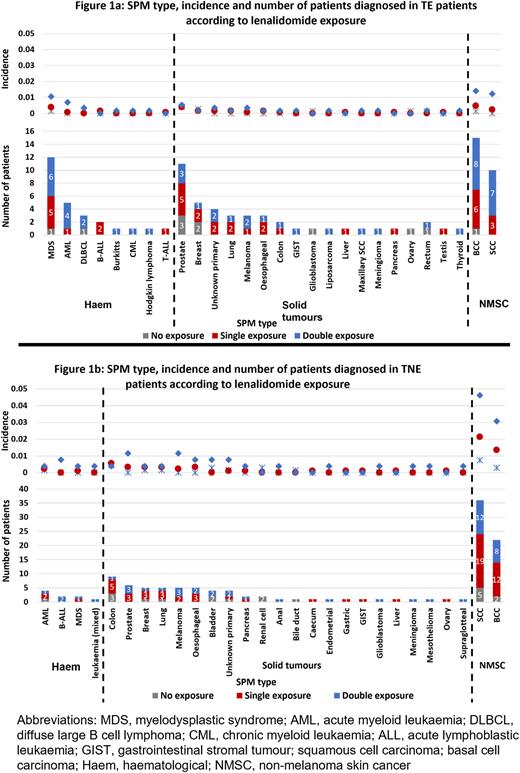
Results 138 TE patients (IR 5.5%) developed an SPM, with 85 diagnosed following maintenance randomization (IR 5.9%). TE patients receiving len maintenance had an SPM incidence of 12.2% at 7 years, compared to 5.8% in those being observed (Pepe-Mori p=0.003). When profiling the SPM type, the incidence of most cancers was greater in the double exposed patients (non-exposed IR 1.6%, single exposed IR 2.9%, double exposed IR 8.3%). Despite this, the actual incidence was <1% for each SPM type in all groups, except for BCC (1.4%), SCC (1.2%) and MDS (1.1%) noted in >1% of double exposed patients. Haematological SPM were almost all confined to the len treated patients, with most in the double exposed group, Figure 1a.
180 TNE patients (IR 9.9%) developed an SPM, with 110 diagnosed following maintenance randomization (IR 13.2%). TNE patients receiving len maintenance had an SPM incidence of 17.1% at 5 years, compared to 10% in those being observed (p=0.09). There was a greater incidence of most SPM types in the double exposed patients (non-exposed IR 2.8%, single exposed IR 6.7%, double exposed IR 18.1%). Skin cancers were common, particularly in the double exposed patients, with an SCC incidence of 4.6% and BCC incidence of 3.1%. No SPM type was diagnosed in >1% of patients who did not receive len. In the double exposed patients, prostate cancer (1.2%) and melanoma (1.2%) were also noted in >1% of patients, Figure 1b. Haematological SPM were rare in TNE patients, with only 8 patients diagnosed, 7 of whom received len.
We also determined the outcome in patients treated with len maintenance. In TE patients, 13 (IR 1.8%) deaths were secondary to an SPM, compared to 121 (IR 16.6%) myeloma related and 18 (IR 2.5%) non-myeloma related deaths. This compared to 2 (IR 0.4%) SPM deaths, 117 (IR 22.6%) myeloma related and 19 (IR 3.7%) non-myeloma related deaths in the observed patients.
In TNE len maintenance patients, 25 (IR 6.1%) died due to a SPM, 133 (32.7%) due to progressive myeloma and 44 (IR 10.8%) due to other causes. In the observed TNE patients, 9 (IR 2.8%) died due to an SPM, 131 (IR 41.5%) due to progressive myeloma and 28 (IR 8.9%) secondary to non-myeloma related issues.
Conclusions SPM incidence was higher in patients treated with len at both induction and maintenance compared to single exposure or no exposure. This was most marked in the TNE patients suggesting an innate risk of SPM development in older patients, particularly skin cancers, possibly impacted by len. Focusing on the maintenance therapy, there was a significant increase in SPM incidence in TE patients treated with len maintenance compared to observation. In TNE patients there was also a numerical increase in SPM incidence that did not reach statistical significance. Deaths due to SPM were rare but increased with lenalidomide maintenance. However, deaths due to myeloma were lower in patients treated with len. We suggest that clinic review of patients receiving maintenance len should include a history focused on identifying possible SPM, so intervention can be made early.
Disclosures
Jones:Janssen: Honoraria. Cairns:Celgene/BMS: Honoraria; Amgen: Research Funding; Takeda: Research Funding. Menzies:Takeda: Research Funding; Celgene/BMS: Research Funding; Amgen: Research Funding. Pawlyn:Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel support; Sanofi: Consultancy, Honoraria; Abbvie: Consultancy. Davies:Takeda, Abbvie, Amgen, BMS/Celgene, Sanofi, GSK, Janssen: Membership on an entity's Board of Directors or advisory committees. Jenner:Pfizer: Consultancy; Janssen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Takeda: Consultancy; GSK: Consultancy. Kaiser:Takeda: Honoraria; Janssen: Honoraria, Research Funding; Karyopharm: Consultancy; GSK: Consultancy; Seattle Genetics: Consultancy; AbbVie: Consultancy; BMS/Celgene: Honoraria, Research Funding; Pfizer: Consultancy. Drayson:Abingdon Health: Current equity holder in private company. Gregory:Janssen: Consultancy; Abbvie: Consultancy. Owen:Astra-Zeneca: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boyd:Janssen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Cook:Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Karyopharm: Consultancy; BMS/Celgene: Consultancy, Research Funding; Amgen: Consultancy. Jackson:BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J and |J: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal